Key Takeaways:
- Federal awards fuel innovation and growth in the biotechnology and life sciences sector, but they require stringent adherence to regulations and the terms of the award.
- The risks of non-compliance include financial penalties, loss of funding, and even legal action.
- An experienced federal award audit provider can improve performance by mitigating risk, adhering to complex regulations, and delivering additional operational and financial insight.
—
The path to groundbreaking biotech and life science innovations is paved with both opportunities and regulatory challenges. Federal awards and grants support the biotech sector’s quest for advancement. However, navigating the maze of compliance requirements demands expertise, precision, and a proactive approach.
Most federal awards require stringent adherence to federal regulations and the terms of the award. The result of non-compliance is severe, and may include financial penalties, the loss of funding, reputational damage, and in the worst circumstances, potential legal action from funding agencies and/or stakeholders.
This is why it is essential to work with an assurance provider with specific knowledge of the biotech/life science landscape. Your audit provider must provide specialized guidance for the sector’s dynamic nature, focusing on risk assessment, control evaluation, and regulatory adherence to safeguard your projects and funding.
Why Program-Specific Audits Matter
In the detail-oriented and highly regulated biotech industry, a generic approach to auditing cannot uncover or address the nuanced complexities of federal funding compliance. Every federal award program has unique regulatory and reporting requirements. A one-size-fits-all approach is likely to miss key details of your specific requirements and may waste essential time and resources on irrelevant matters.
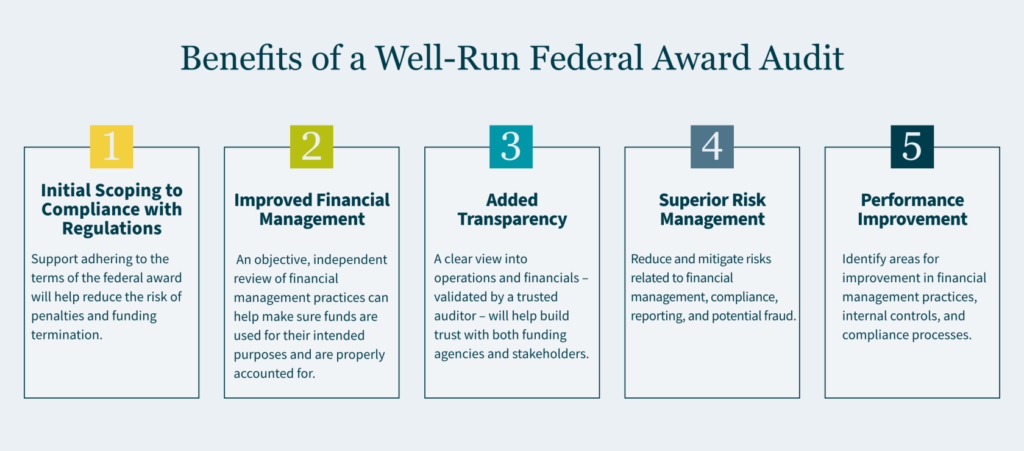
The benefits of a well-run audit by a seasoned provider should include:
- Compliance with Regulations: Support adhering to the terms of the federal award will help reduce the risk of penalties and funding termination.
- Improved Financial Management: An objective, independent review of financial management practices can help make sure funds are used for their intended purposes and are properly accounted for.
- Added Transparency: A clear view into operations and financials – validated by a trusted auditor – will help build trust with both funding agencies and stakeholders.
- Superior Risk Management: Reduce and mitigate risks related to financial management, compliance, reporting, and potential fraud.
- Performance Improvement: Identify areas for improvement in financial management practices, internal controls, and compliance processes.
Optimal Federal Award Audit Approach
Conducting a federal award audit for a biotechnology company involves several key steps to ensure compliance with federal regulations and the terms of the award. Our audit process is differentiated by its collaborative, transparent, and client-focused methodology. We start with an in-depth evaluation of your unique needs and the specifics of your programs to craft a tailored strategy. Throughout the audit journey, we provide open communication, delivering insights and updates to keep you informed and engaged. Our final deliverable is a comprehensive report that addresses compliance, empowers strategic decision-making, and program enhancement.
The following is our outline of the process:
- Analysis of Award Terms: Review of the terms and conditions of the federal award to understand the requirements related to financial management, reporting, and compliance.
- Collect Documentation: Assemble all relevant documentation, including financial records, invoices, receipts, and others.
- Financial Record Review: Examination of your company’s financial records to confirm that expenses are properly documented and allocated.
- Compliance Assessment: Evaluation of your company’s compliance with the federal award requirements, including allowable costs, cost-sharing, reporting, and other terms in the award agreement.
- Perform Testing: Select a sample of transactions and expenses to test for compliance with federal regulations and the award terms. This may include testing for allowability, allocability, and reasonableness of costs.
- Document Findings: Document the results of the audit, including any findings of non-compliance or areas for improvement.
- Prepare Audit Report: Prepare a formal audit report summarizing the audit findings, conclusions, and recommendations for corrective action, if necessary.
- Communicate Results: Discuss the audit findings with the company’s management and provide them with an opportunity to respond and address any issues identified during the audit.
- Follow-Up: Monitor the company’s corrective actions and follow up to identify and resolve issues in a timely manner.
- Federal Agency Report: Submit the audit report to the appropriate federal agency responsible for overseeing the award, along with any recommendations for further action.
Benefits of an Experienced Biotechnology Audit Provider
When selecting a federal audit provider, it is important to identify a firm with a dedicated biotechnology and life sciences practice with professionals actively serving the industry. Your provider should bring a wealth of experience and a multidisciplinary team that specializes in the financial intricacies and regulatory frameworks governing the biotech industry.
At MGO, our professionals are not only auditors but also advisors versed in the nuances of biotech funding and federal award compliance. We understand the operational, financial, and compliance pressures you face and offer bespoke auditing solutions that reflect the latest regulatory standards and industry best practices.
We can provide support in a number of areas:
- Compliance Assurance: Leveraging our deep understanding of federal regulations and the biotech landscape, we can guide your organization through all compliance requirements, protecting you from potential penalties and financial discrepancies.
- Risk Management: Our targeted audits help identify and address risks specific to your federal awards, enhancing the efficiency and impact of your funded programs.
- Strategic Guidance: We go beyond compliance, offering insights and strategies to optimize the management and performance of your federally funded projects, aligning them with your mission and federal objectives.
Let’s Get to Work
In the biotech sector, where federal awards catalyze innovation, MGO is uncommonly positioned to guide organizations through the complexities of program-specific audits for federal awards, determining compliance, and the strategic allocation and utilization of federal funds. MGO can help you optimize the management and performance of your federally funded projects, leading to greater efficiency, impact, and innovation.
Discover how MGO’s specialized auditing services can empower your biotech organization to achieve compliance, efficiency, and innovation. Contact us today to explore how we can support the success and compliance of your federally funded programs, allowing you to focus on advancing biotechnology’s boundaries.